Qualification & Validation
Cleaning Method Design and Cleaning Validation – A Challenge for Both New and Existing Facilities
Cleaning has been one of the most common challenges both new and existing facilities face. A lack of understanding of cleaning systems in facilities can result in warning letters by regulatory compliance, overuse of resources to obtain a repeatably clean system, or inability to expand the production capacity to meet the market demand. As a result, cleaning validation is under constant scrutiny by regulatory authorities, a critical aspect of GMP and quality control.
Issuance of warning letters due to noncompliance in cleaning validation continues to be a significant issue for many manufacturers, despite the availability of detailed guidance or resources on cleaning validation. A few of the common cleaning failures are using incorrect cleaning agents, the unreliability of the chemical dosing, incomplete SOPs, inconsistencies in the systems to repeatably clean, etc.
On the other hand, new facilities tend to overlook the significance of a good cleaning validation master plan and design of CIP cycles. This results in high consumption of water and chemicals, inefficient CIP cycles, ineffective cleaning validation strategy, and the inability of this plan to accommodate for future expansions. However, manufacturers’ can follow certain guidelines to improve the efficiency of their CIP cycles as well as to obtain a repeatably clean system.
Typical Solutions for Existing Facilities
The facilities can make use of bench-scale data obtained from developmental cleaning studies to improve the cleanability of the system, shorten the cleaning cycle times and improve the turnaround time. Developmental cleaning studies are bench-scale tests that evaluate the performance of a particular set of parameters, visual, gravimetric, and rinse sampling assessment methods [1]. These bench-scale studies have been beneficial in determining the optimal cleaning chemistry, temperature, cycle duration, and chemical concentration.
These studies use the soil present at the production facility to obtain more targeted data. Mostly, cleaning agent manufacturers’ have helped their clients run developmental studies. However, they lack the resources to do a design space exploration to identify the optimum processing parameters as it requires a significant investment of time. Using the optimal cleaning parameters obtained from these developmental cleaning studies can eliminate the failures caused by using legacy CIP cycles.
Another challenge faced by manufacturers who are looking to expand the production capacity of their existing facilities is the inability of the cleaning systems to accommodate for the expansion. The capacity constraints imposed by the cleaning system can be significantly improved by optimizing the cleaning cycles using the processing parameters obtained from the developmental cleaning studies [2].
In addition to using developmental studies to obtain optimum processing parameters, these studies are important to understand the chemistry of the soil. The chemistry and nature of the soil present at the facility are essential to ensure that a proper cleaning agent is being used. The most appropriate cleaning agent can be selected by comparing different cleaning agents and their interaction with the soil.
Typical Solutions for New Facilities
New manufacturers are often challenged with a lack of infrastructure, resources, and data to develop a robust Cleaning Validation Plan (CVP). During the initial stages of the design, new facilities can benefit by creating a Cleaning Validation Plan (CVP) that is more adaptable and well-planned. This can help reduce the amount of time spent validating similar systems by incorporating a grouping strategy. Furthermore, they can also optimize their validation procedure for future system upgrades and reduce the amount of validation assessment required for new products with the proper use of non-specific analytical methods.
The new facilities can benefit from an efficient design of CIP cycles and cleaning studies. The cleaning studies can help determine the optimum cleaning processing parameters using design space exploration and also provide a cleaning duration estimate to serve as a starting point. These studies should incorporate a worse-case residue program to assess the efficiency of the new cleaning cycles. It is strongly recommended for the newly built facilities to conduct degradation studies before choosing the chemical agent. These studies help to assess how the soil interacts with different chemical agents.
A Different Approach to Addressing the Challenges
Recognizing the challenges that existing and new facilities face, Hyde created the Hyde Analytical Lab to efficiently develop cleaning cycles and improve the efficiency of the CIP systems. Using the above-mentioned solutions, we have repeatedly improved the cleanability of the clients’ system, shortened their cleaning cycle times, and improved their turnaround time.
With over a decade of experience, the Hyde Analytical Lab has repeatedly demonstrated that the parameters developed in our lab are transferrable to full-scale cleaning in manufacturing environments. Using lab scale results, we can successfully identify facility’s potential CIP failures prior to the implementation at the site. The lab has a standard set of experimental tests to evaluate and filter out the possible cleaning issues for almost any situation.
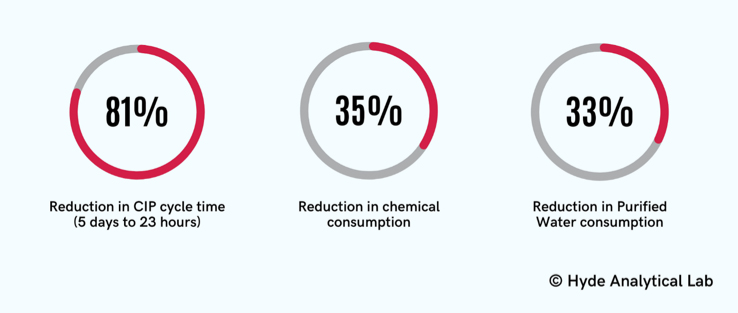
The bench-scale tests conducted for one of our clients’ facility using specialized equipment achieved a 100% CIP cycle success rate. Using the newly designed CIP cycles, resulted in a reduction of CIP cycle times from five days to 23 hours. These new cycles also resulted in a 35% reduction in chemical consumption and a 33% reduction in purified water consumption. In addition, the CIP cycles were harmonized for multiple products for the facility.
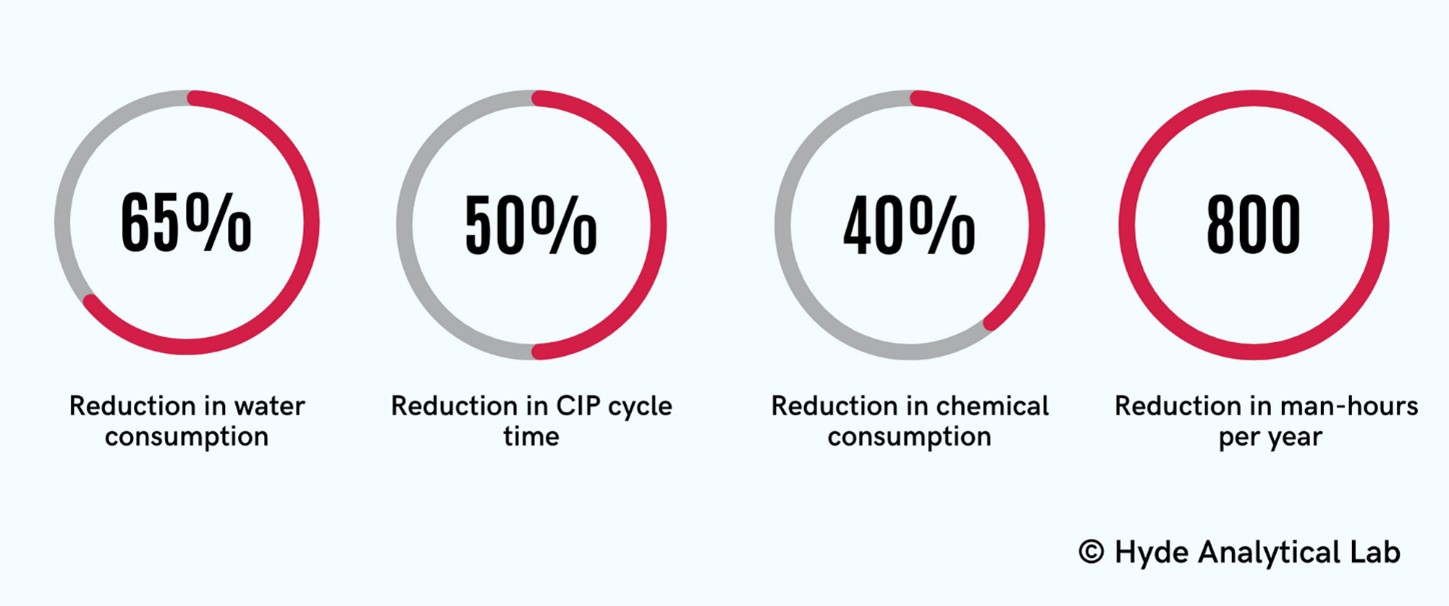
Clients often implement overkill CIP cycles, and the Analytical Lab has also helped several clients improve the efficiency of their CIP systems. The optimal cleaning parameters identified in the Lab have been successful in supporting numerous clients facing the challenge of increasing their production capacity. For example, water shortages and longer cleaning times were production bottlenecks for a client. The Lab team identified a goal to improve the cleaning times by 50% with a corresponding 50% reduction in water consumption in order to prepare for the expanded production demands. At the end of the project, we successfully reduced 40% chemical consumption using dissolution testing, 65% reduction in water consumption surpassing our goal, 50% reduction in cleaning times using the optimal cleaning parameters, and 800 man-hour reductions in cleaning setup and execution time per year.
REFERENCES
- Digital Image Processing for Bench Scale Cleanability Studies
- Application of Lean Six Sigma to Optimize a Legacy Cleaning Process
- Online Total Organic Carbon (TOC) as a Process Analytical Technology for Cleaning Validation Risk Management
- Translating laboratory-developed Visual Residue Limits to Process Area Applications


